Alternatively, if the claim relates to
a category that is excluded from
patentability but “clearly does not seek
to tie [it] up” a streamlined analysis can
be used in step 2a. An example of such
a claim provided by the USPTO refers
to an artificial hip prosthesis coated
with a naturally occurring mineral. This
use of the naturally occurring product
does not claim the naturally occurring
mineral per se, just its use with the
prosthesis and is therefore held to
represent subject matter that is not
excluded from patentability.
However, assuming the analysis
at step 2a comes out as “yes”, the
analysis must proceed to step 2b.
In step 2b the test is whether any
part or combination of parts of the
claim amounts to “significantly more”
than the exception itself. This means
that “the claim describes a product
or process in a meaningful way,
such that it is more than a drafting
effort designed to monopolise the
exception”. Unfortunately, all of the
examples provided to date as to
what might be “significantly more”
relate to mechanical or electronic
fields of technology (although the July
2015 update promises that further
biodiagnostic related examples will
be forthcoming). The USPTO does
note that the steps of “administering”
(e.g. a compound) or “determining”
(e.g. a concentration of analyte)
are not sufficient to satisfy the
requirements of “significantly more”.
Biodiagnostic cases are struggling in
the wake of this new approach. So,
what can applicants in this field do?
The analysis provided in the USPTO
interim guidance appears to be
especially concerned with preventing
applicants from trying to monopolise
subject matter, such as natural
laws, that is excluded from patent
protection. One option for applicants
in the biodiagnostics field may be
to argue that the claims relate to
practical applications of a natural law
and do not seek a monopoly in the
natural law per se. For this to work
it is likely that the claims will need
to include active steps, especially
definite steps of treatment or
making a diagnosis (which will need
to amount to more than “thinking
about the results”). Sensible fall back
positions may include specifying
the methods by which those
treatment or diagnostic steps are
carried out, e.g. specifying the use
of specific antibodies or a particular
type of assay. Of course, this may
lead to narrower claims and these
limitations will need to be drafted
carefully.
As personalised medicine becomes
increasingly important, biodiagnostic
and biomarker testing is becoming
critical for effective diagnosis and
treatment of patients. Unfortunately,
due to the significant cost required
to establish the safety and efficacy
of products before they can reach
the market, companies simply
cannot afford to develop these
products without patent protection.
Consequently, there is a real risk that
these developments in US case law
could lead to fewer new products
reaching patients. For this reason,
a European approach, which leaves
doctors and medical practitioners
free to treat or diagnose their
patients as they see fit, while
protecting the products associated
with those methods, seems to be
significantly preferable. Recently the
USPTO’s stance has been challenged
by amicus curiae briefs from
interested groups around the world,
so perhaps in time common sense
will prevail.
13
To find out more
contact Helen Henderson
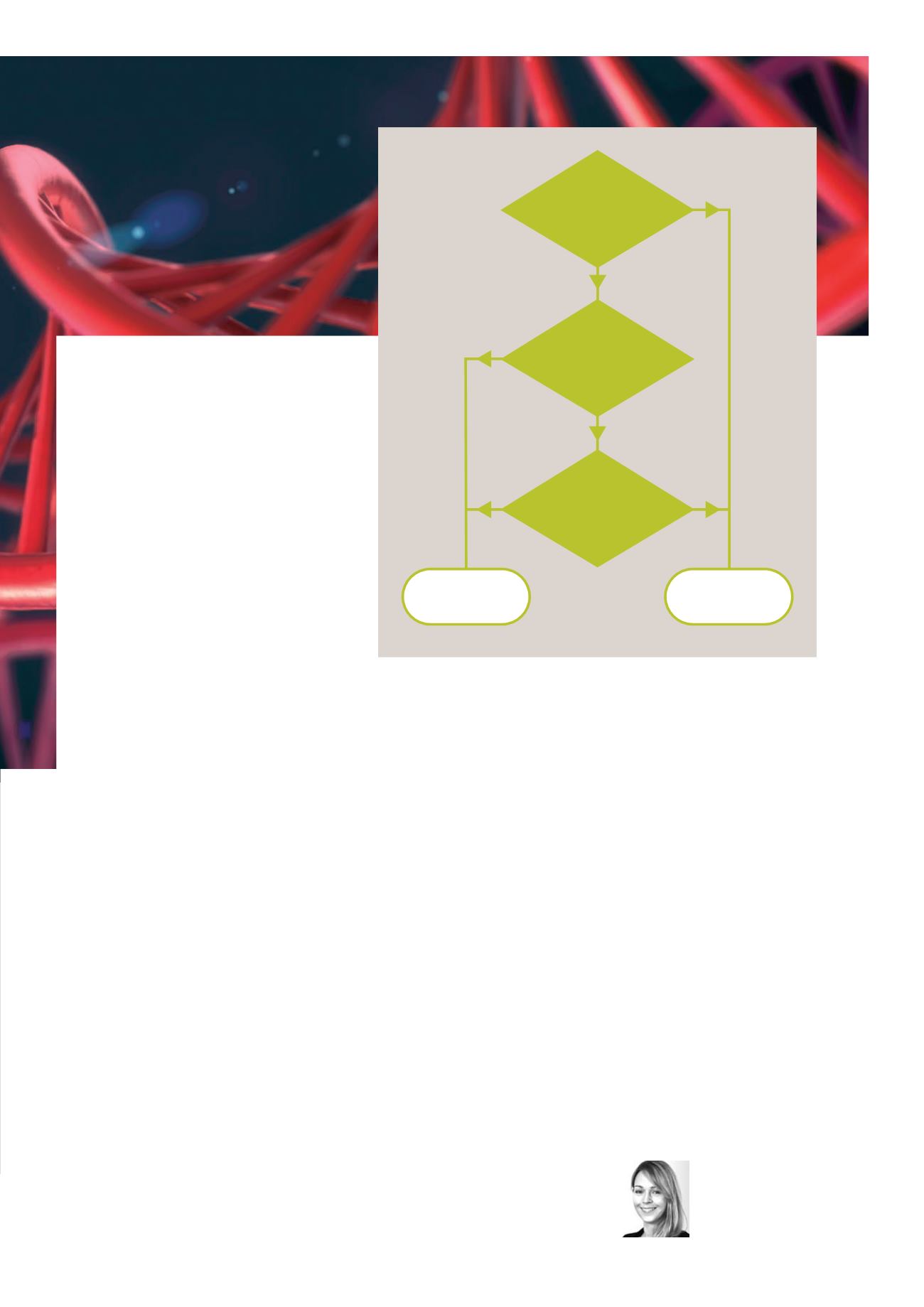
Step 1
IS THE CLAIM TO A PROCESS,
MACHINE OR COMPOSITION OF
MATTER?
Step 2a
IS THE CLAIM DIRECTED TO
A LAW OF NATURE, A NATURAL
PHENOMENON OR AN ABSTRACT
IDEA (JUDICIALLY RECOGNIZED
EXCEPTIONS)?
Step 2b
DOES THE CLAIM RECITE
ADDITIONAL ELEMENTS THAT
AMOUNT TO SIGNIFICANTLY MORE
THAN THE JUDICIAL
EXCEPTION?
CLAIM QUALIFIES AS
ELIGABLE SUBJECT MATTER
UNDER 35 USC 101
CLAIM IS NOT ELIGABLE
SUBJECT MATTER UNDER
35 USC 101
NO
NO
NO
YES
YES
YES


